There are added electronelectron repulsions in the valence shell. The new valence shell is held closer to the nucleus resulting in a smaller radius for the cation.

How Many Valence Electrons Are In Fluorine Clutch Prep
Valence Electrons And Ionic Compounds Video Khan Academy
Illustrated Glossary Of Organic Chemistry Octet Rule
The most reactive kind of metallic element is an alkali metal of group 1 eg sodium or potassium.
Fluorine valence electrons. Of the main group elements fluorine has the highest electronegativity EN 40 and cesium the lowest EN 079. Fluorine participates in the formation of bonds through valence electrons. Valence Electrons of all the elements in the Periodic Table in Graph and Table format Complete information about all the properties of elements using Graphs and Tables Interactive Dynamic Periodic Table Periodic Table Element Comparison Element Property trends and complete information about the element - Facts How to Locate on Periodic Table History Abundance Physical Properties.
Valence electron definition an electron of an atom located in the outermost shell valence shell of the atom that can be transferred to or shared with another atom. Electrons in Neutral Atom Valence Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li Beryllium 4 2 Lose 2 Be2 Boron 5 3 Lose 3 B3 Carbon 6 4 Gain or Lose 4 C4or C4-Nitrogen 7. We can use these values to predict what happens when certain elements combine.
Covalent chemical bonds involve the sharing of a pair of valence electrons by two atoms in contrast to the transfer of electrons in ionic bonds. The Role of Nonbonding Electrons in the VSEPR Theory. If yes you must have observed the pattern in which these 52 cards are grouped in Club Diamond Spade and Heart.
Our goal however isnt predicting the distribution of valence electrons. For example oxygen and boron Draw Lewis dot diagrams of the following atoms. The fluorine valence electrons pull as far apart as possible or 180 giving this compound a linear shape.
How did Rutherford figure out the structure of the atom without being able to see it. Such bonds lead to stable molecules if they share electrons in such a way as to create a noble gas configuration for each atom. Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic tableThe halogen elements are fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts.
This is because such an atom has only a single valence electron. Therefore group number fourteen is also called the carbon group. Have you ever played cards.
The valence electrons on the central atom in both NH 3 and H 2 O should be distributed toward the corners of a tetrahedron as shown in the figure below. The first element of group-14 is carbon. A A sulfur atom in which all 6 of its valence electrons have been fully ionized away by six fluorine atoms or b A sulfur atom with a stable highly symmetric 12-electron valence shell that is both created and stabilized by six octahedrally located fluorine atoms each of which covalently shares an electron pair with the central sulfur atom.
The chemical symbol for Fluorine is F. It can either share electrons with a neighboring atom to form a covalent bond or it can remove electrons from another atom to form an ionic bond. While playing cards we often deal with games that include getting cards from other players to fulfil the games objective like grouping four sevens four aces etc.
Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Electrons are arranged in shells or energy levels. An anion has a larger radius than the neutral atom because it gains valence electrons.
The number of valence electrons in an atom governs its bonding behavior. In chemistry and atomic physics an electron shell may be thought of as an orbit followed by electrons around an atoms nucleusThe closest shell to the nucleus is called the 1 shell also called the K shell followed by the 2 shell or L shell then the 3 shell or M shell and so on farther and farther from the nucleusThe shells correspond to the principal quantum numbers n. Carbon will then have five valence electrons its four and the one its sharing with fluorine.
Fluorine is a halogen element and its symbol is F. The nucleus is composed of protons and neutrons. NONMETALS A nonmetal tends to attract additional valence electrons to attain a full valence shell.
So the valence electrons of carbon are 4. Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. Valence Electrons Valency of Hydrogen H.
Thus fluorine has seven valence electrons. Element Atomic Atomic Mass Protons Neutrons Electrons Lewis Dot Helium 2 4 2 2 2 He Oxygen 8 16 8 8 8 O Fluorine 9 19 9 10 9 F. The following video shows this.
Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core. A fluorine atom has seven valence electrons. The valence electrons of all the elements of group-13 including boron are three.
If you add another fluorine atom to make BeF 3 the furthest the valence electron pairs can get from each other is 120 which forms a trigonal planar shape. The total number of electrons present in the valence shell of an atom is called valence electrons and there are a total of seven electrons present in the valence shell of fluorine 2s 2 2p 5. Valence Electrons Valency of Carbon C.
Valence electrons are the total number of electrons present in the outermost shell of an atom ie. The electron configuration of carbon shows that carbon has a total of four electrons in its final orbit. The most reactive kind of nonmetal is a halogen such as fluorine or chlorine.
They were given the name halogen from the Greek roots hal- salt and -gen to produce because they all produce sodium salts of similar properties of which. This indicates that fluorine has a high tendency to gain electrons from other elements with lower electronegativities. Valence electrons are the outer electrons in an atom that participate in chemical reactions and determine chemical changes to atoms and molecules.
Nitrogen 7 14 7 7 7 N Silicon 14 28 14 14 14 Si. Atoms are neutral so this means sodium also has 11 electrons. Depending on your level of Chemistry it is probably easier to think of them as particles orbiting the nucleus.
Lewis dot diagrams are a way to indicate the number of valence electrons around an atom. Valence electrons are the outermost electrons and are the ones involved in bondingSodium has 11 electrons. The valence electrons for a neutral atom are always definite it cannot be varied more or less in any condition for a particular atom and may or may not be equal to its valency.
Hydrogen Ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort to attain a stable octet of electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. A cation has a smaller radius than its neutral atom because it loses valence electrons.
Its atomic number is 11 so it has 11 protons. If it shares one electron with a carbon atom which has four valence electrons the fluorine will have a full octet its seven electrons plus the one it is sharing with carbon. This article discusses in detail the valence electrons of fluorine.

Electron Configurations The Periodic Table

State The Number Of Valence Electrons The Group Number And The Period Number Of Each

How Many Valence Electrons Does Fluorine Have Lacylearning

What Are Properties Of The Group Of Elements On The Periodic Table That Have 7 Valence Electrons A High Reactivity Metallic B High Reactivity Nonmetallic C Low Reactivity Metallic D Low Reactivity
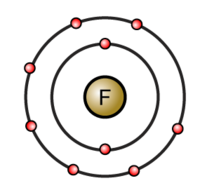
Why Do Two Fluorine Atoms Bond Together Socratic

Does Fluorine Have 5 Or 7 Active Valence Electrons Chemistry Stack Exchange
How Many Valence Electrons Are In Fluorine Quora

How Many Dots Would Be Placed Around Fluorine In A Lewis Electron Dot Diagram A 2 B 7 C 9 D 19 Study Com

